Our series of patient profiles were developed to mimic real-world case studies to illustrate the clinical value of a test capable of individually identifying more high-risk HPV types. Check out the following patient profiles to learn how using an HPV test with extended genotyping can optimize risk stratification for your patients during routine cervical cancer screening.1,3-5
Offer your patients the personalized management they deserve


Meet Tianna*, a 35-year-old who tested positive for HPV 31
HPV 31 poses a higher risk of precancer as compared to HPV 18.1 HPV tests that group HPV 31 with other HPV types in one pooled result may underestimate a woman’s risk for cervical precancer and cancer due to HPV 31.1,2
By using a test that can individually identify HPV 31, you offer your patients a more precise cervical cancer risk assessment compared to an HPV test with grouped results.3

Meet Grace*, who has a persistent infection with the same HPV type
Persistent infection with the same high-risk HPV type causes the cells of the cervix to change and is the number one risk factor for cervical precancer and cancer, regardless of the type.3-6
A test that individually identifies more high-risk HPV types expands your ability to determine if an HPV-positive result is due to a type-specific persistent infection, so you can focus on your patients most at risk for progression.3,4

Meet Maya*, who cleared one HPV type and then tested positive for another
Testing positive for a different high-risk HPV type at two consecutive tests resets the risk for cervical precancer or cancer to that of having a new infection and is lower risk compared to having the same type.5
A test capable of individually identifying more high-risk HPV types expands your ability to determine if an HPV positive result at follow-up is due to a different HPV type.3
Learn more about extended genotyping with the BD Onclarity™ HPV Assay
This form is only intended for use by healthcare professionals seeking additional information about BD products and solutions. Patients seeking health and treatment information should contact their healthcare professional and can find additional resources from ACOG and the American Cancer Society.
Do not submit protected health information (PHI) or patient information (PII) through this form. If you do submit PHI or PII, your question may not be answered, as the information will be deleted to protect your privacy.
The 2019 ASCCP Risk-based Management Consensus Guidelines for the management of cervical cancer screening abnormalities recommend 1 of 6 clinical actions, according to the risk of cervical precancer and cancer (CIN3+ risk).7
But how precise is the HPV test that you are using?
To make more informed decisions you need to be empowered with more information.
By using a test capable of individually identifying more high-risk HPV types you can more precisely determine a woman’s risk of cervical precancer and cancer compared to a test with a large, pooled "HPV Other" result – and ultimately offer your patients like Tianna, Grace and Maya a more informed, personalized management strategy. 1,3-5
BD Onclarity™ HPV Assay is the first FDA-approved HPV assay to test for an extended set of genotypes individually and adds value to your practice by:8-13
- Individually identifying the top 3 HPV types that contribute the most to cervical precancer and cancer – HPV 16, 31, and 18.8,14
- Tracking a persistent infection with the same high-risk HPV type – the number one risk factor for cervical precancer and cancer – for HPV 16, 18, 31, 45, 51 and 52.4,6,8
- Identifying when one HPV type is replaced by another between tests versus a persistent infection with the same HPV type.4,8
- Detecting lower risk types in strategic grouped results.8
*Names and/or medical information presented on this page do not represent real people or clinical information.
ASCCP, American Society for Colposcopy and Cervical Pathology; CIN3+, cervical intraepithelial neoplasia grade 3, adenocarcinoma in situ, or cancer; FDA, Food and Drug Administration; HPV, human papillomavirus.
1. Stoler MH et al. Gynecol Oncol. 2019,153(1):26–33.
2. Egemen D et al. J Low Genit Tract Dis. 2020;24(2):132–43.
3. Bonde J et al. J Low Genit Tract Dis. 2020;24(1):1–13.
4. Bonde J et al. J Low Genit Tract Dis. 2021;25(1):27–37.
5. Elfgren K et al. Am J Obstet Gynecol. 2017;216(3):264.e1–7.
6. Bodily J, Laimins LA. Trends Microbiol. 2011;19(1):33–9.
7. Perkins RB et al. J Low Genit Tract Dis. 2020;24(2):102–31.
8. BD Onclarity™ HPV Assay US Package Insert [8089894].
9. digene® HC2 High-Risk HPV DNA Test Package Insert.
10. Aptima™ HPV Assay US Package Insert.
11. Cervista™ HPV HR US Package Insert.
12. cobas® HPV for 4800 System US Package Insert.
13. cobas® HPV for 6800/8800 System US Package Insert.
14. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in United States of America. Summary Report 10 March 2023. Accessed August 11, 2023.
ASCCP's recommended management based on calculated risk of CIN3+
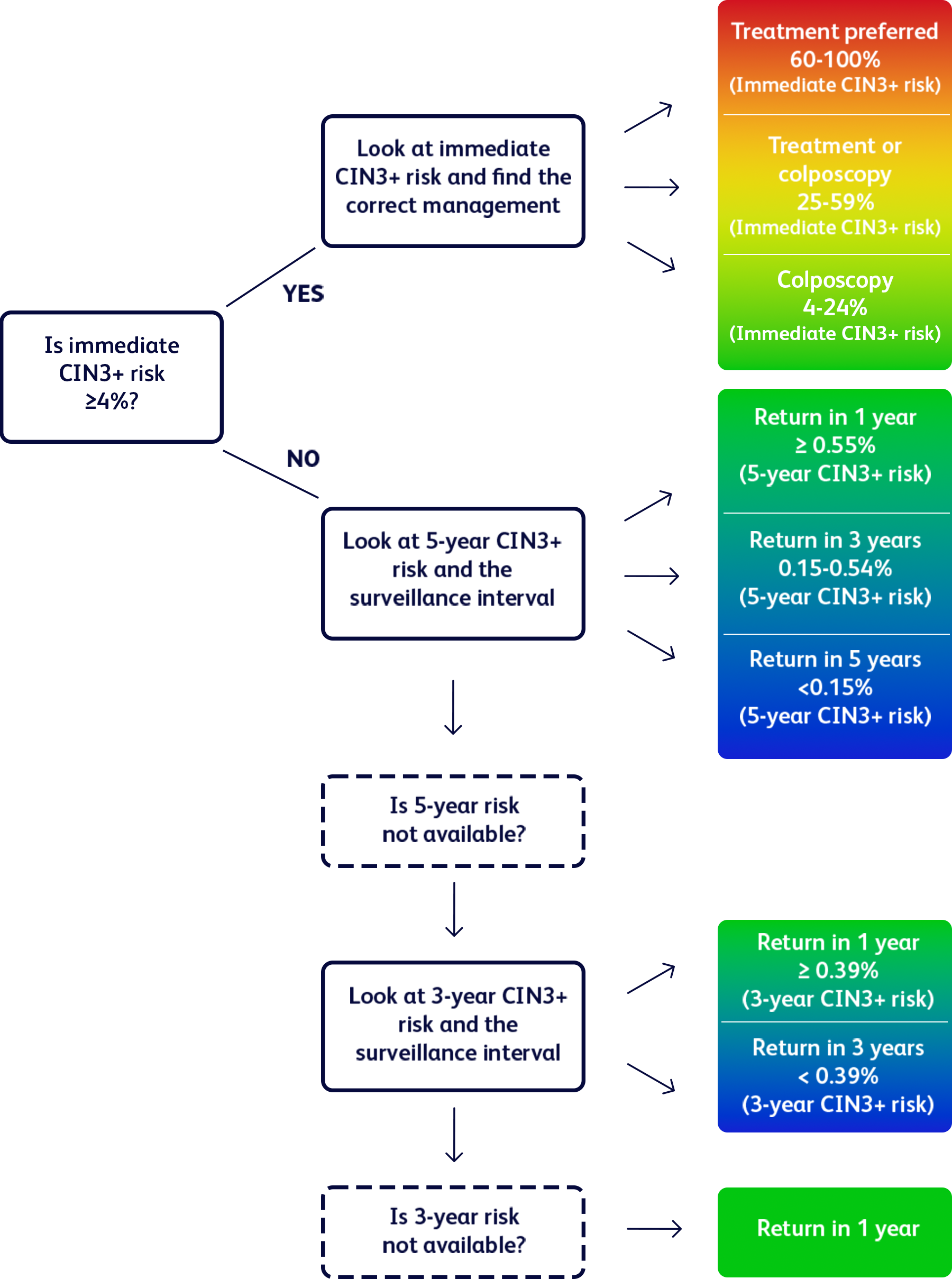
Adapted from Egemen D, et al. J Low Genit Tract Dis. 2022;26(3):195-201.
ASCCP, American Society for Colposcopy and Cervical Pathology; CIN3+, cervical intraepithelial neoplasia grade 3, adenocrarcinoma in situ, or cancer; HPV, human papillomavirus.




























