Meet Grace*
- 41 years old
- No medical conditions
- Is a busy entrepreneur, but always on top of her healthcare appointments
- Has cervical cancer screening done about every 5 years
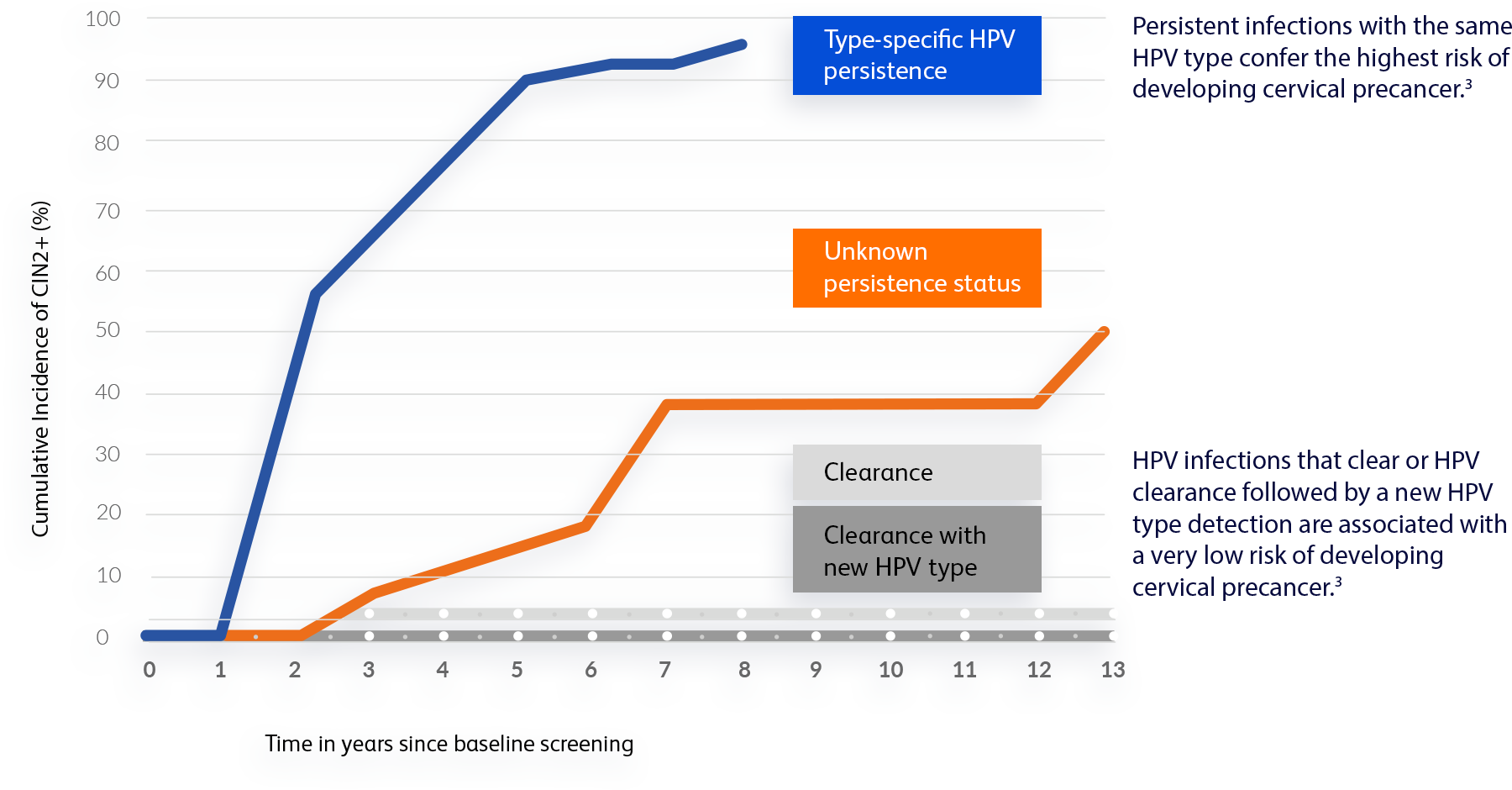
Last year, Grace found out she tested positive for HPV 52 and her OB/GYN strongly recommended follow-up testing in one year for surveillance to see if the infection would clear or if it would persist.
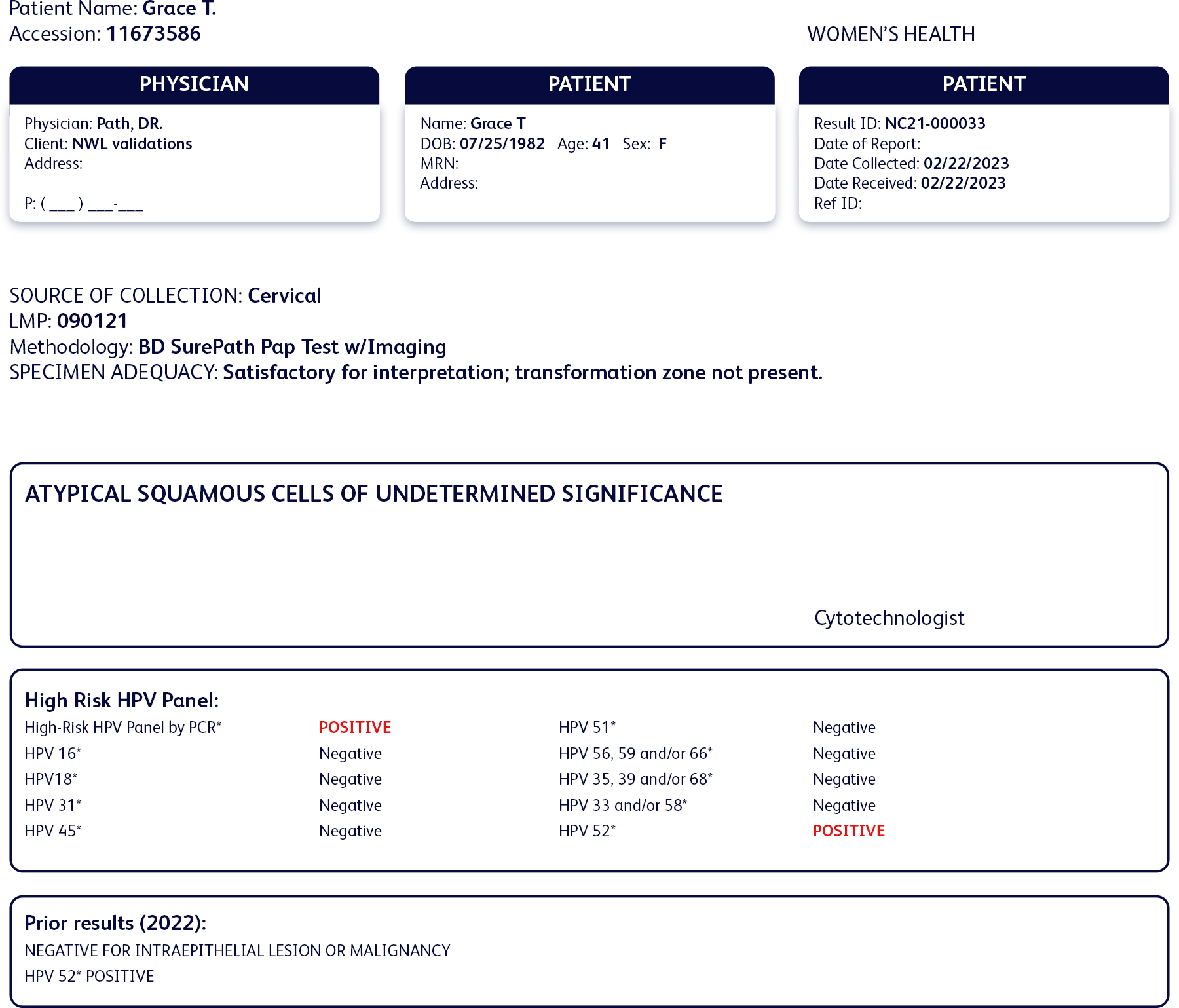
Cervical cancer screening history
Past test results:
- Normal cytology
- Positive for HPV 52
- Negative for all other high-risk HPV types
Current results:
- ASC-US cytology
- Positive for HPV 52
- Negative for all other high-risk HPV types
Grace’s new test results showed atypical squamous cells of undetermined significance (ASC-US) and a persistent infection with HPV 52.

“When my OB/GYN told me that I was still positive for HPV 52, I was shocked. A persistent HPV infection can be serious and lead to cervical cancer. I’m so thankful that my doctor used this test and followed-up.”