Meet Maya*
- 45 years old
- No medical conditions
- Trusts her OB/GYN and is aware of the benefits of regular cervical cancer screening
- Has cervical cancer screening test every 5 years
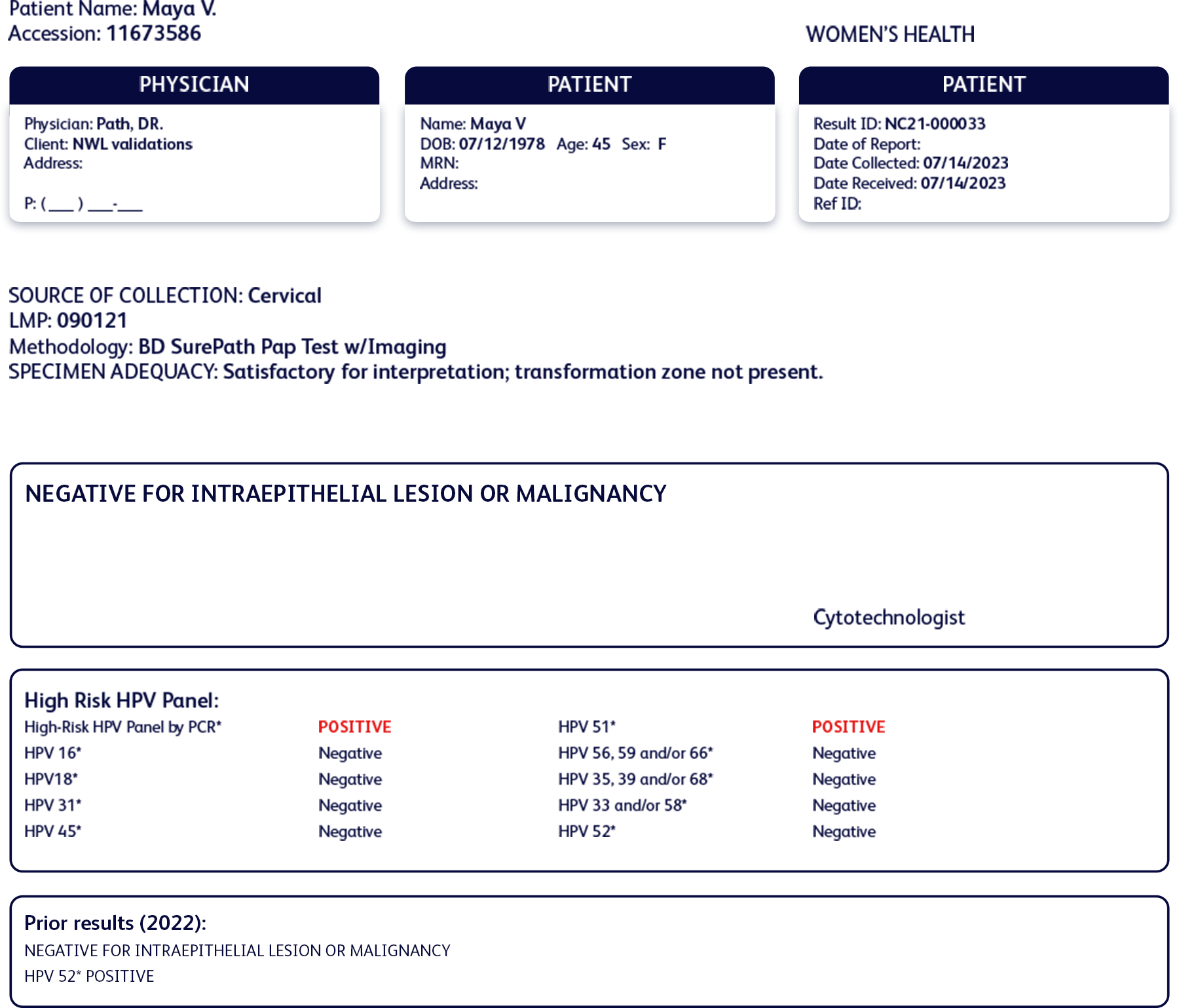
Maya had a positive test result with HPV 52 one year ago and has come back for a follow-up HPV test as instructed by her OB/GYN.
Cervical cancer screening history
Past test results:
- Normal cytology
- Positive for HPV 52
- Negative for all other high-risk HPV types
Current results:
- Normal cytology
- Positive for HPV 51
- Negative for HPV 52 and all other high-risk HPV types
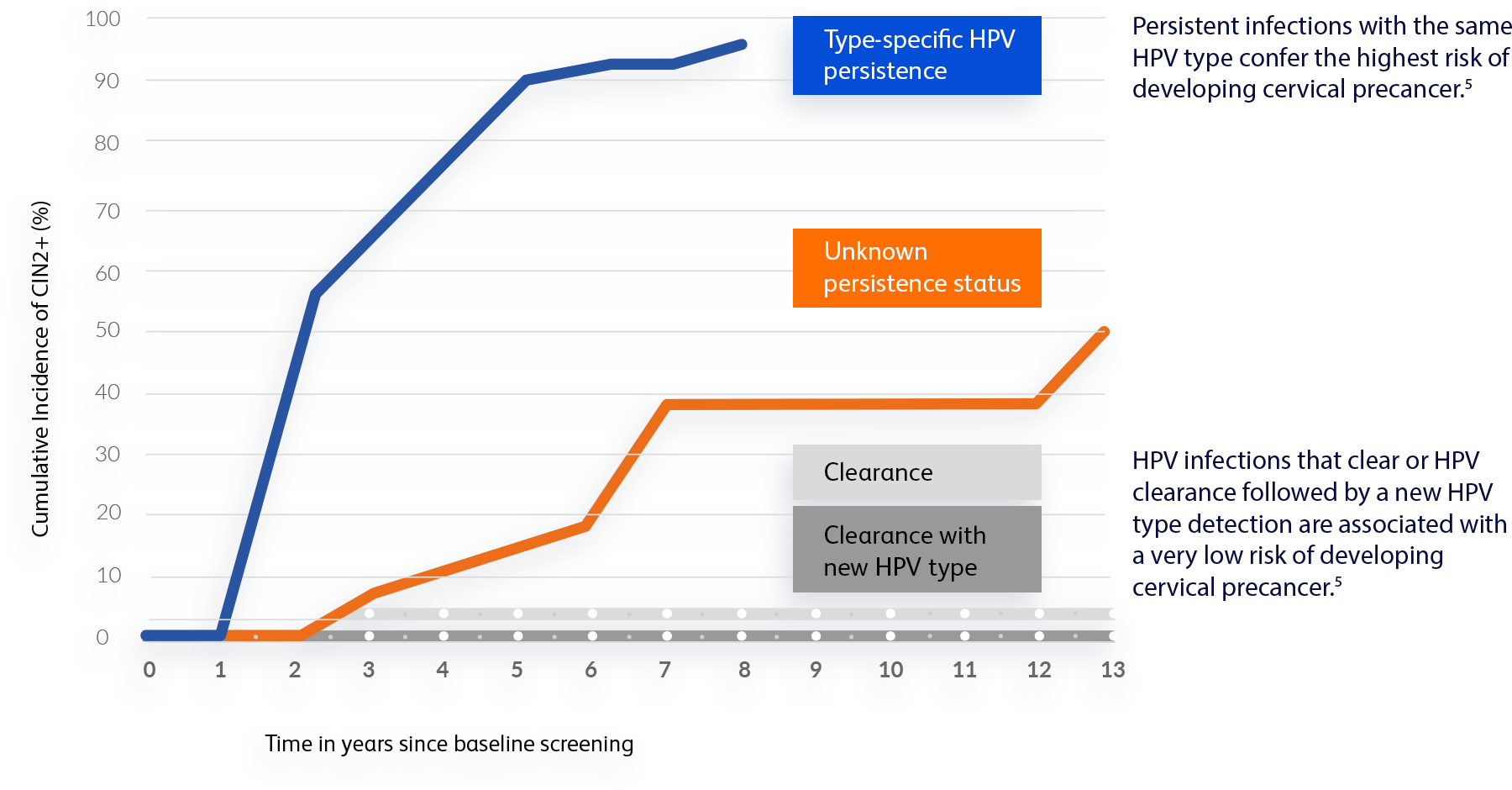
1-year after her initial positive HPV test, she cleared the infection with HPV 52 and tested positive for a different type, HPV 51.

“At first, having a positive result with a different HPV type didn’t make sense to me. Then, my OB/GYN explained that HPV can sometimes be undetectable for years before showing on a test. I’m now reassured because it actually means my risk is lower than if I had the same HPV type as last year.”