Meet Tianna*
- 35 years old
- No medical conditions
- Lives a healthy lifestyle and believes in the importance of cervical cancer screening
- Has had Pap smears every 3 years from age 21 to 29 and co-testing with HPV and Pap every five years starting at age 30
Tianna recently moved to a new city and found a new OB/GYN where she was offered an HPV test that provided more detailed results.
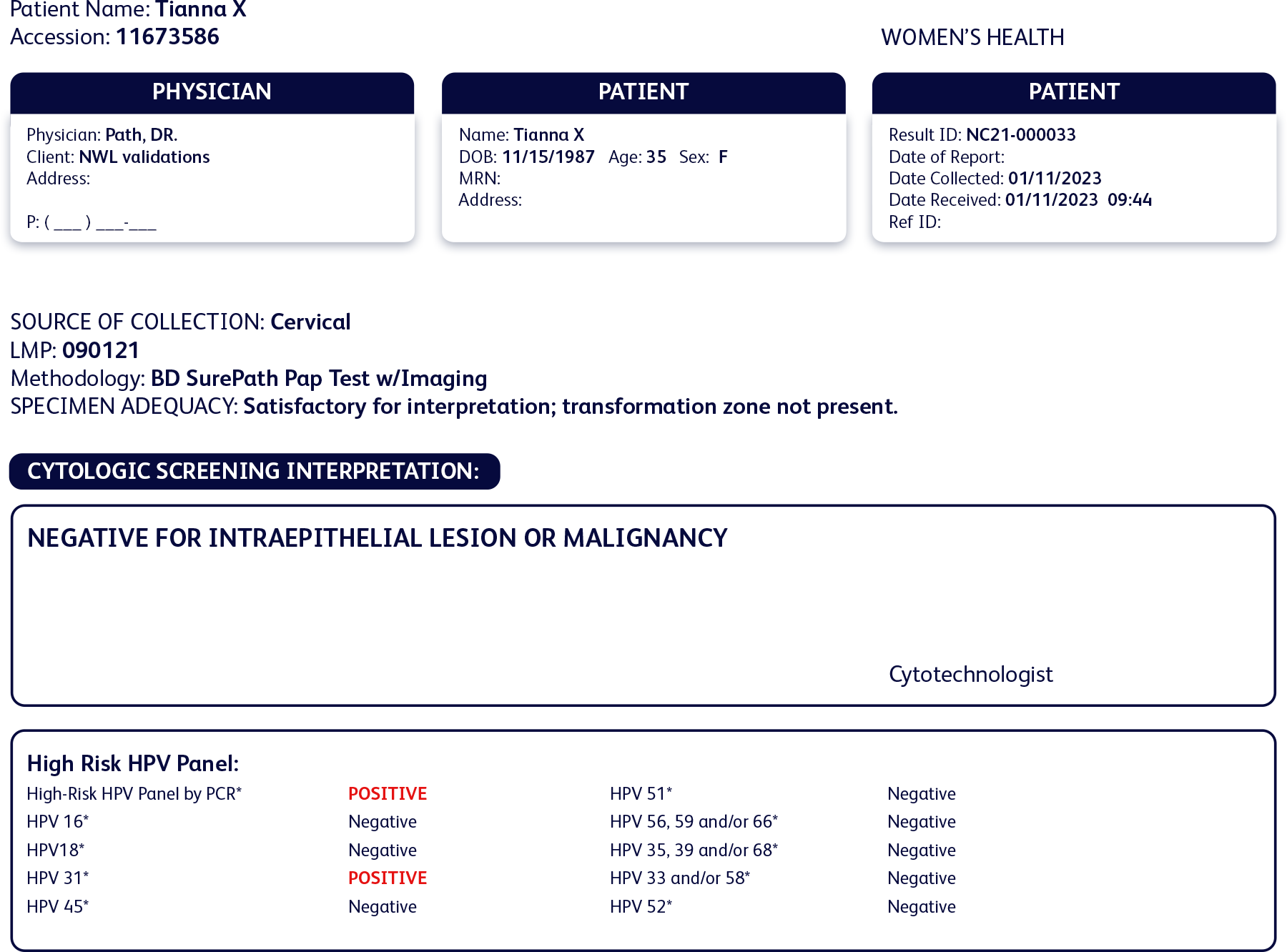
Cervical cancer screening history
Past test results:
- Normal cytology
- Negative for HPV 16 and 18
- Negative for high-risk HPV pool
Current results:
- Normal cytology
- Negative for HPV 16 and 18 and all other high-risk types
- Positive for HPV 31
Her cytology results were once again normal, but her HPV test results showed an infection with HPV 31.

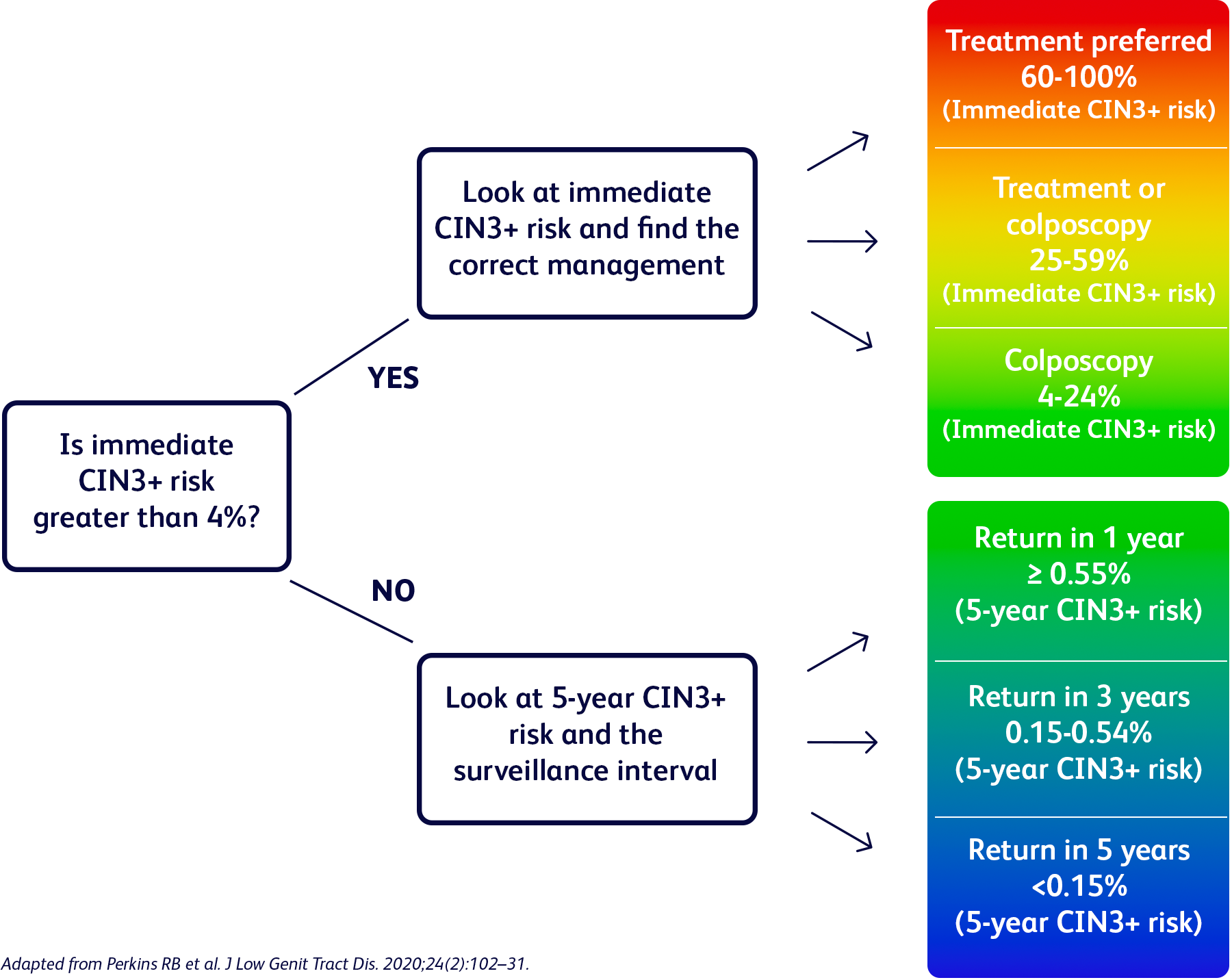
“I was worried when I first found out that I had HPV 31, one of the most risky types of HPV, and what it meant for my risk of cervical precancer. But my OB/GYN was very supportive and we were quickly able to discuss a plan”*